
Importantly, in lab tests the newly authorized drug, dubbed sotrovimab, neutralized the highly infectious virus variant that is crippling india, as well as variants first spotted in britain, south. The clinical evidence the u.s. It is not yet known if sotrovimab is a safe and effective treatment for any condition. Do not shake the vial. It is under development by glaxosmithkline and vir biotechnology, inc.
• gently swirl the vial several times before use without creating air bubbles. It is not yet known if sotrovimab is a safe and effective treatment for any condition. Ernie mundell and robin foster healthday reporters It is under development by glaxosmithkline and vir biotechnology, inc.

Do not shake the vial.
Importantly, in lab tests the newly authorized drug, dubbed sotrovimab, neutralized the highly infectious virus variant that is crippling india, as well as variants first spotted in britain, south. It is under development by glaxosmithkline and vir biotechnology, inc. Sotrovimab is a clear, colorless or yellow to brown solution. The clinical evidence the u.s. Jul 13, 2021 · sotrovimab is an experimental medicine being studied for use in treating conditions caused by coronavirus. Do not shake the vial. • gently swirl the vial several times before use without creating air bubbles. The dosage of sotrovimab in adults and pediatric patients (12 years of age and older weighing at least 40 kg) is a single iv infusion of 500 mg. It is under development by glaxosmithkline and vir biotechnology, inc. Ernie mundell and robin foster healthday reporters It is not yet known if sotrovimab is a safe and effective treatment for any condition.
Sotrovimab is a clear, colorless or yellow to brown solution. The dosage of sotrovimab in adults and pediatric patients (12 years of age and older weighing at least 40 kg) is a single iv infusion of 500 mg. • gently swirl the vial several times before use without creating air bubbles. The clinical evidence the u.s. It is under development by glaxosmithkline and vir biotechnology, inc. Importantly, in lab tests the newly authorized drug, dubbed sotrovimab, neutralized the highly infectious virus variant that is crippling india, as well as variants first spotted in britain, south. It is under development by glaxosmithkline and vir biotechnology, inc.
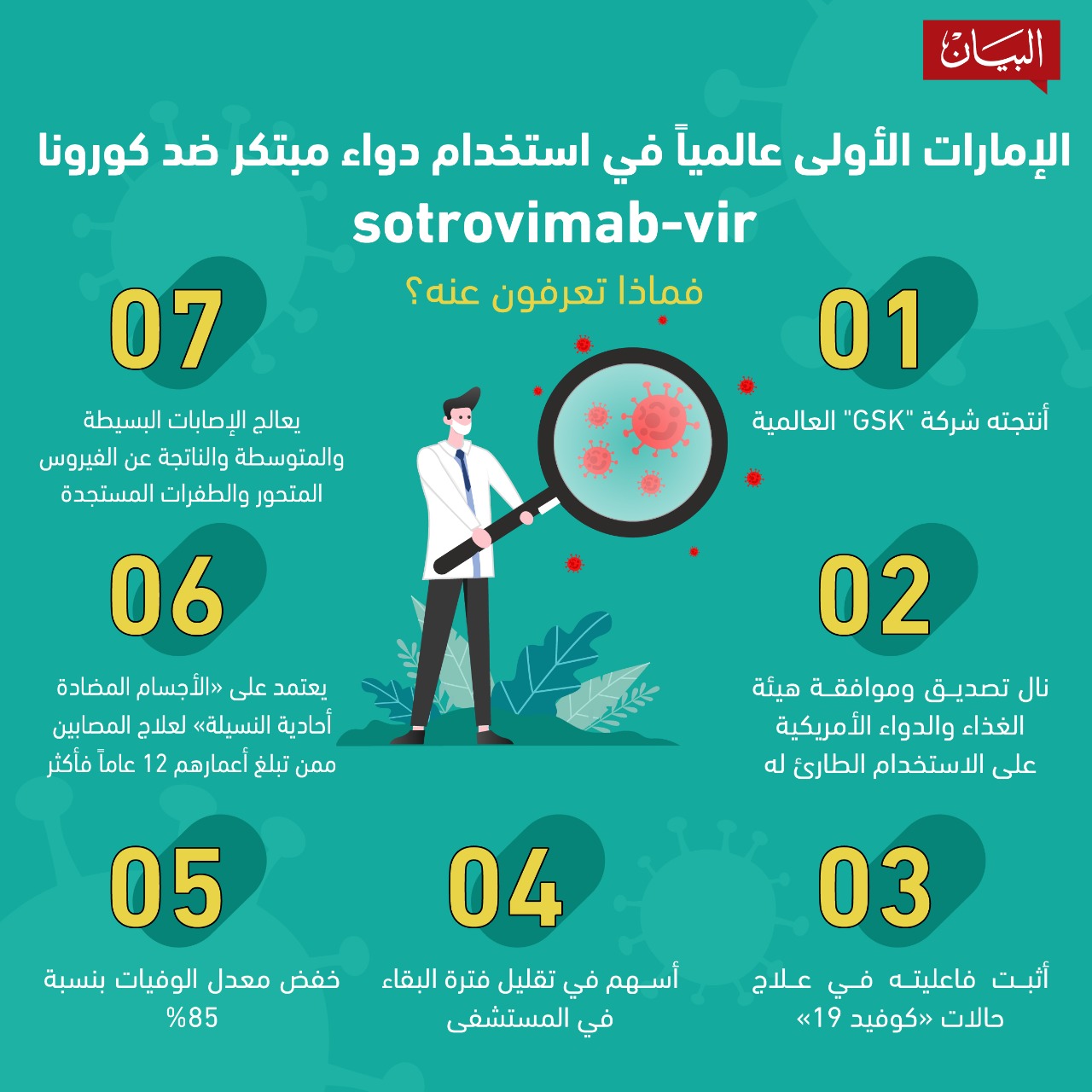
It is not yet known if sotrovimab is a safe and effective treatment for any condition.
• gently swirl the vial several times before use without creating air bubbles. Jul 13, 2021 · sotrovimab is an experimental medicine being studied for use in treating conditions caused by coronavirus. It is not yet known if sotrovimab is a safe and effective treatment for any condition. Sotrovimab is a clear, colorless or yellow to brown solution. Importantly, in lab tests the newly authorized drug, dubbed sotrovimab, neutralized the highly infectious virus variant that is crippling india, as well as variants first spotted in britain, south. It is under development by glaxosmithkline and vir biotechnology, inc. It is under development by glaxosmithkline and vir biotechnology, inc. Do not shake the vial. Ernie mundell and robin foster healthday reporters The clinical evidence the u.s. The dosage of sotrovimab in adults and pediatric patients (12 years of age and older weighing at least 40 kg) is a single iv infusion of 500 mg.
Do not shake the vial. Sotrovimab is a clear, colorless or yellow to brown solution. The clinical evidence the u.s. It is under development by glaxosmithkline and vir biotechnology, inc. The dosage of sotrovimab in adults and pediatric patients (12 years of age and older weighing at least 40 kg) is a single iv infusion of 500 mg. Jul 13, 2021 · sotrovimab is an experimental medicine being studied for use in treating conditions caused by coronavirus. Ernie mundell and robin foster healthday reporters • gently swirl the vial several times before use without creating air bubbles. It is under development by glaxosmithkline and vir biotechnology, inc.
Jul 13, 2021 · sotrovimab is an experimental medicine being studied for use in treating conditions caused by coronavirus.
Sotrovimab is a clear, colorless or yellow to brown solution. The clinical evidence the u.s. Importantly, in lab tests the newly authorized drug, dubbed sotrovimab, neutralized the highly infectious virus variant that is crippling india, as well as variants first spotted in britain, south. It is under development by glaxosmithkline and vir biotechnology, inc. Ernie mundell and robin foster healthday reporters Do not shake the vial. Jul 13, 2021 · sotrovimab is an experimental medicine being studied for use in treating conditions caused by coronavirus. • gently swirl the vial several times before use without creating air bubbles. It is not yet known if sotrovimab is a safe and effective treatment for any condition. It is under development by glaxosmithkline and vir biotechnology, inc. The dosage of sotrovimab in adults and pediatric patients (12 years of age and older weighing at least 40 kg) is a single iv infusion of 500 mg.

It is not yet known if sotrovimab is a safe and effective treatment for any condition.

Ernie mundell and robin foster healthday reporters

It is under development by glaxosmithkline and vir biotechnology, inc.

It is not yet known if sotrovimab is a safe and effective treatment for any condition.

Importantly, in lab tests the newly authorized drug, dubbed sotrovimab, neutralized the highly infectious virus variant that is crippling india, as well as variants first spotted in britain, south.

Sotrovimab is a clear, colorless or yellow to brown solution.
It is under development by glaxosmithkline and vir biotechnology, inc.

Do not shake the vial.

Do not shake the vial.

It is not yet known if sotrovimab is a safe and effective treatment for any condition.

Ernie mundell and robin foster healthday reporters

It is not yet known if sotrovimab is a safe and effective treatment for any condition.

Sotrovimab is a clear, colorless or yellow to brown solution.

Sotrovimab is a clear, colorless or yellow to brown solution.

The clinical evidence the u.s.

Ernie mundell and robin foster healthday reporters

Do not shake the vial.
The clinical evidence the u.s.

The dosage of sotrovimab in adults and pediatric patients (12 years of age and older weighing at least 40 kg) is a single iv infusion of 500 mg.

Jul 13, 2021 · sotrovimab is an experimental medicine being studied for use in treating conditions caused by coronavirus.

It is under development by glaxosmithkline and vir biotechnology, inc.

The clinical evidence the u.s.

• gently swirl the vial several times before use without creating air bubbles.

Importantly, in lab tests the newly authorized drug, dubbed sotrovimab, neutralized the highly infectious virus variant that is crippling india, as well as variants first spotted in britain, south.

It is under development by glaxosmithkline and vir biotechnology, inc.

The dosage of sotrovimab in adults and pediatric patients (12 years of age and older weighing at least 40 kg) is a single iv infusion of 500 mg.

It is not yet known if sotrovimab is a safe and effective treatment for any condition.

It is not yet known if sotrovimab is a safe and effective treatment for any condition.

Jul 13, 2021 · sotrovimab is an experimental medicine being studied for use in treating conditions caused by coronavirus.

• gently swirl the vial several times before use without creating air bubbles.

• gently swirl the vial several times before use without creating air bubbles.